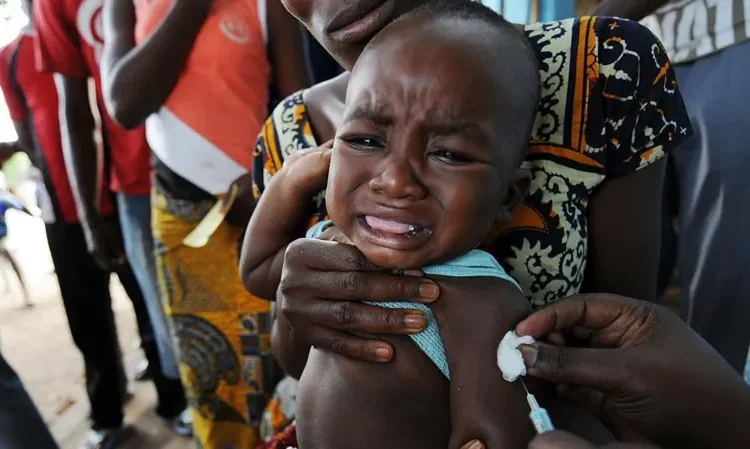
The World Health Organization (WHO) has condemned a US-funded hepatitis B vaccine trial in Guinea-Bissau as “unethical,” following plans to test the effects of delaying the birth-dose vaccine for thousands of newborns.
The halted study had proposed giving one group of babies the hepatitis B vaccine at birth, while delaying vaccination for another group until six weeks old. The WHO said this approach exposed infants to “potentially irreversible harm,” as the birth-dose vaccine is a proven, life-saving intervention used for more than three decades in over 115 countries.
Robert F. Kennedy Jr., who has publicly questioned vaccine safety, had spearheaded the US health department’s support for the study. The WHO raised significant concerns about the trial’s scientific justification, ethical safeguards, and adherence to established human research standards.
Hepatitis B is highly prevalent in Guinea-Bissau, with more than 12% of adults chronically infected, and smaller studies suggesting rates as high as 20%. The virus can be transmitted from mother to child at birth in 70–95% of cases, and infection in newborns often leads to lifelong chronic disease, including cirrhosis and liver cancer.
“The trial sought to withhold a proven intervention from some newborns, which is only acceptable when no effective treatment exists,” the WHO said. It recommends all infants receive the hepatitis B vaccine within 24 hours of birth to prevent lifelong infection.
Public outrage in Guinea-Bissau led the government to suspend the project last month. Former Health Minister Magda Robalo criticized the trial, saying, “Guinea-Bissauans are not guinea pigs.”
The study, led by Danish researchers, was set to involve 14,000 babies. Guinea-Bissau currently administers the vaccine at six weeks, with plans to introduce the birth-dose nationwide by 2028 to align with global standards — a move the WHO supports.
Critics have questioned the ethics of targeting African newborns for research, particularly given the known benefits of early vaccination. The controversy has reignited debates around vaccine safety, ethics in global health research, and the role of US-funded studies abroad.